Creating economic motives to reduce carbon emissions
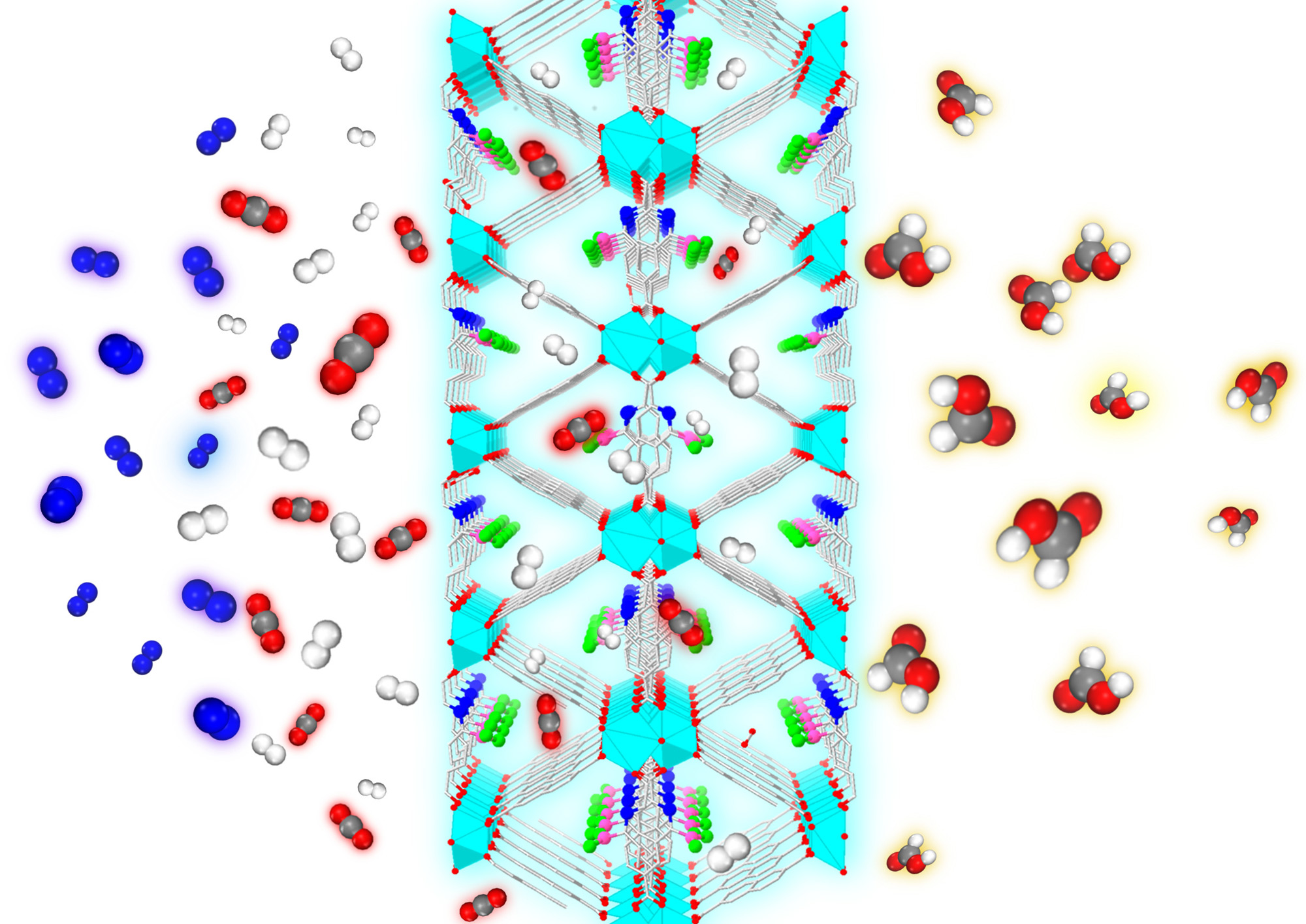
are exposed to a metal-organic framework. Only the CO2 and H2 enter the MOF,
which rejects the N2. The catalytic sites within the framework convert
the CO2 to formic acid (red, gray and white), a chemical precursor to methanol.
Coal-fired electrical power plants produce almost 70 percent of the carbon dioxide emissions created by electrical power plants in the United States – and in 2018 they produced almost 30 percent of the available electricity. Coal remains the primary fuel in 18 states. No alternative power source now exists at that scale. Proposed solutions abound for reducing or eliminating CO2 emissions to curb global warming, but any new technology needs to be as cost-effective and undisruptive as possible to be widely adopted.
Reducing CO2 emissions costs money. Existing technology to capture CO2 in exhaust produced by coal-fired electrical power plants costs an estimated $600 per ton of carbon removed, based on a process called capture and sequestration – trapping CO2 in liquid, heating it to release it from the liquid, and pumping it underground. The process consumes a significant part of the power produced by the plant, raising the price of electricity.
J. Karl Johnson, Pitt CRC Associate Director and Professor in the Department of Chemical and Petroleum Engineering, and other Pitt CRC collaborators work to develop technology that not only reduces emissions but creates incentives to reduce emissions by transforming captured CO2 into valuable fuels and chemicals, offsetting capture costs while creating a possible profit motive.
Johnson’s team works with a class of nanomaterials called metal organic frameworks (MOF), made up of metal ions and organic linkers, combined to produce porous absorbent crystalline structures. MOFs can be manipulated to separate and interact with specific molecules, making it possible to design efficient catalysts to both capture and transform CO2. In 2018, Johnson and his team succeeded in designing two particularly promising MOFs able to bend CO2 molecules to make them react easily with hydrogen. The research was described in the September 2018 Catalysis Science & Technology.
Johnson explains, "We identified two MOFs that have the potential to be used for both CO2 capture from exhaust gas, and for catalytic conversion of CO2 into valuable chemicals. Within the lattice formed by the MOFs, the catalytic reaction of CO2 and hydrogen combine to produce formic acid, which is used to synthesize methanol and other chemicals. This points the way to the ultimate goal of finding a low-energy, low-cost MOF able to separate carbon dioxide from a mixture of gases and prepare it to react with hydrogen. With the right MOF, we could capture CO2 from flue gas at power plants or directly from the atmosphere. We continue searching for that MOF, and we are narrowing the search.”
Johnson used CRC resources to build a database of groups of atoms within molecules – moieties – to identify catalysts best able to bind to a customized MOF. Modeling each member of the database using quantum mechanics required upwards of 300,000 computing hours on CRC resources.
Chris Wilmer, assistant professor in the Department of Chemical and Petroleum Engineering, also focuses on the intersection of science and economics in carbon capture. He models polymer composites called mixed matrix membranes, built upon MOFs.
Wilmer and team develop computational models of mixed matrix membranes to predict those most capable of storing high volumes of CO2. A paper escribing the computational models was recently published in the journal Energy & Environmental Science – “High-throughput computational prediction of the cost of carbon capture using mixed matrix membranes” (Wilmer and his team collaborated on the paper with Jan Steckel, research scientist at the U.S. Department of Energy’s National Energy Technology Laboratory).
As with Johnson’s work, evaluating the number of potential combinations requires significant CRC computing resources. Databases of hypothetical and real MOFs revealed more than one million potential mixed matrix membranes. Wilmer’s team compared each material and evaluated them based on variables such as pressure and temperature conditions, with the goal of identifying specific membranes that capture CO2 at the least cost.
Wilmer explains. “Analyzing that large group yielded 1,153 mixed matrix membranes with a carbon capture cost of less than $50 per ton removed, which is roughly half the existing cost estimates for other MOF-based technology. The potential exists for creating an economically affordable and efficient means of CO2 capture at coal power plants here and throughout the world in places where coal-fired power plants remain common.”
Johnson hopes economically viable capture processes will lead to scaled-up technology in the near future. “Any realistic assessment of the energy landscape shows that it would be technically unfeasible and economically a disaster to move headlong to a zero-carbon emissions society. Carbon transformation technology based on MOFs could be a stop gap for the next 50 years, giving us time to ramp up renewable energy sources.”
Contact:
Brian Connelly
Pitt Center for Research Computing
bgc14@pitt.edu
412-383-0459
